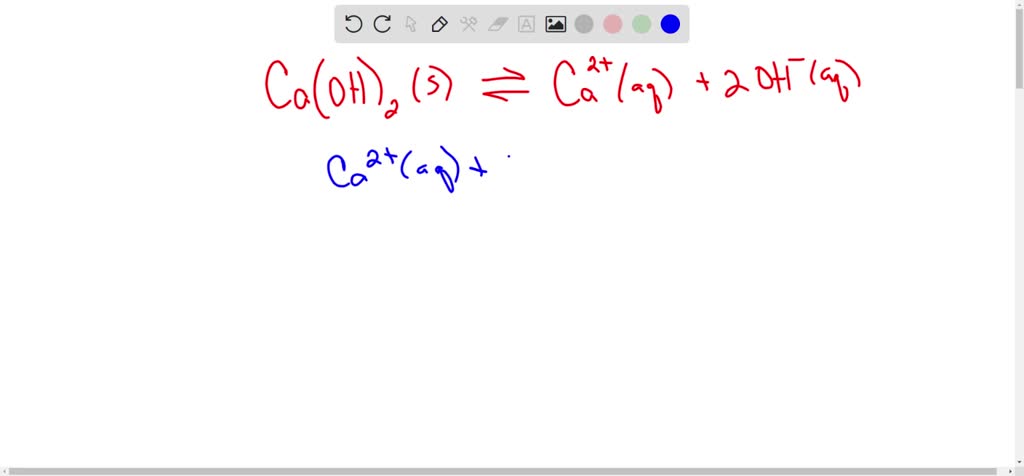
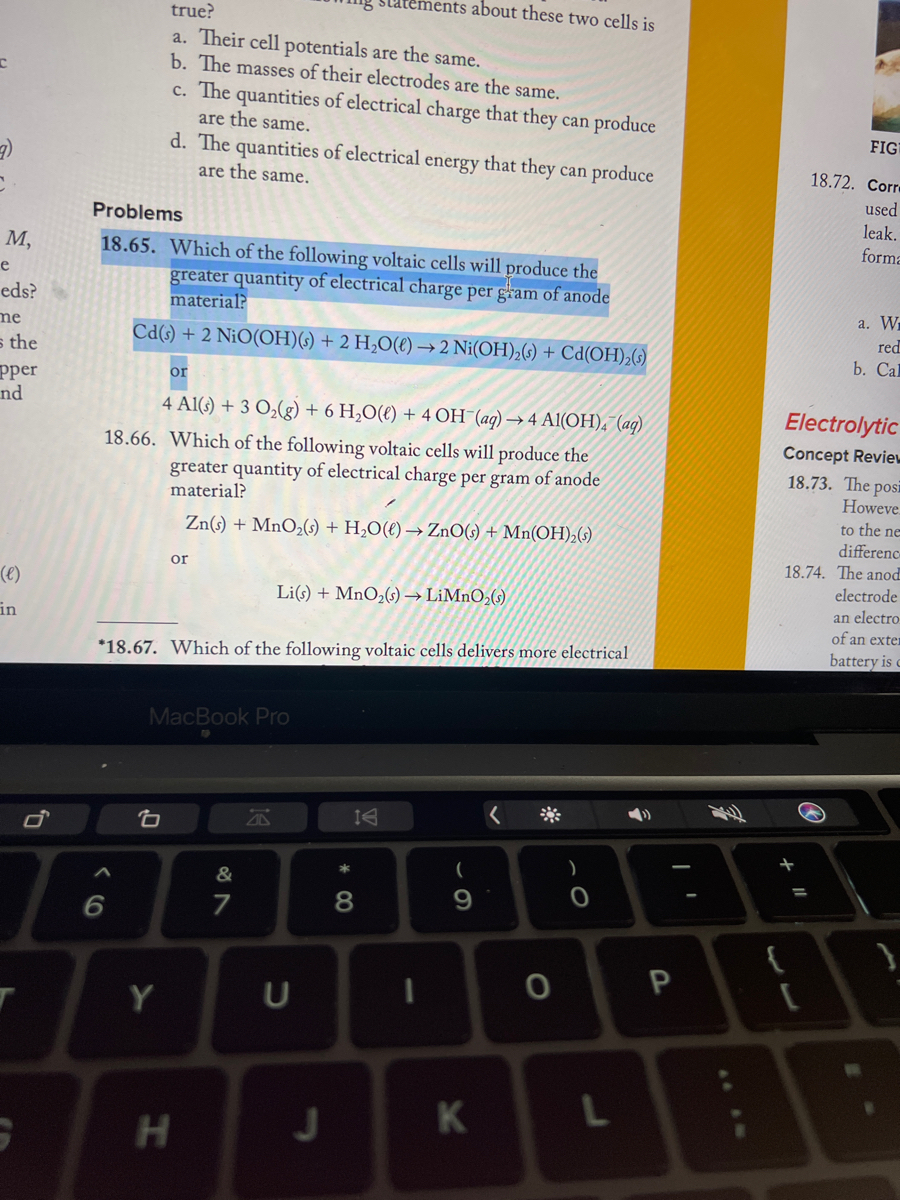
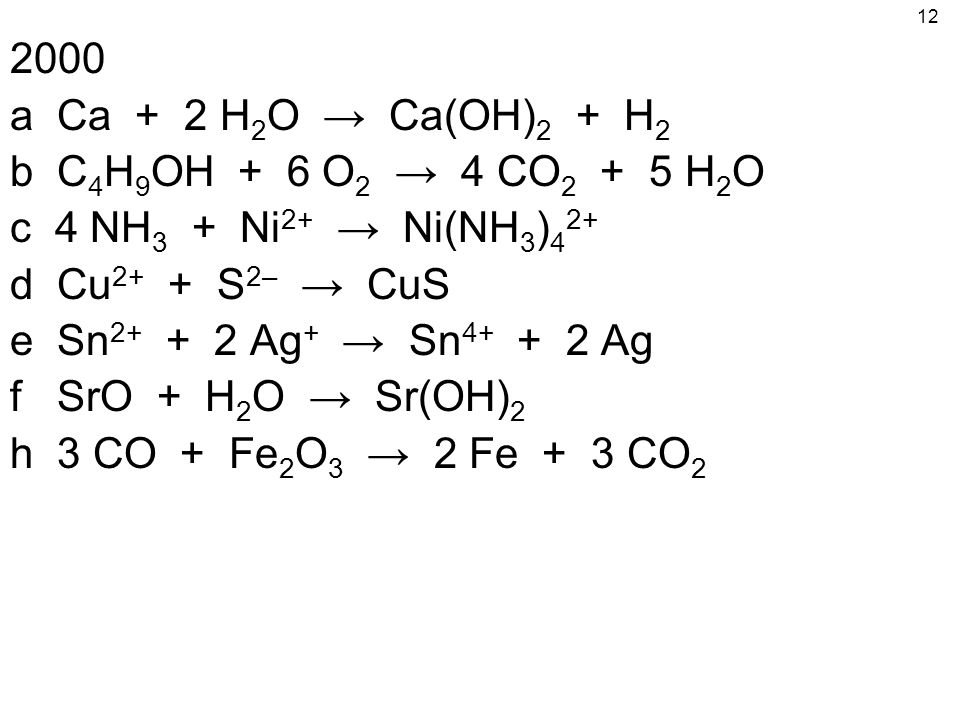SOLVED: Consider these compounds: A. Fe2S3 B. Caz(PO4)2 C. Ni(CN)2 D. Ca(OH) 2 Complete the following statements by entering the letter(s) corresponding to the correct compound(s). (If more than one compound fits the

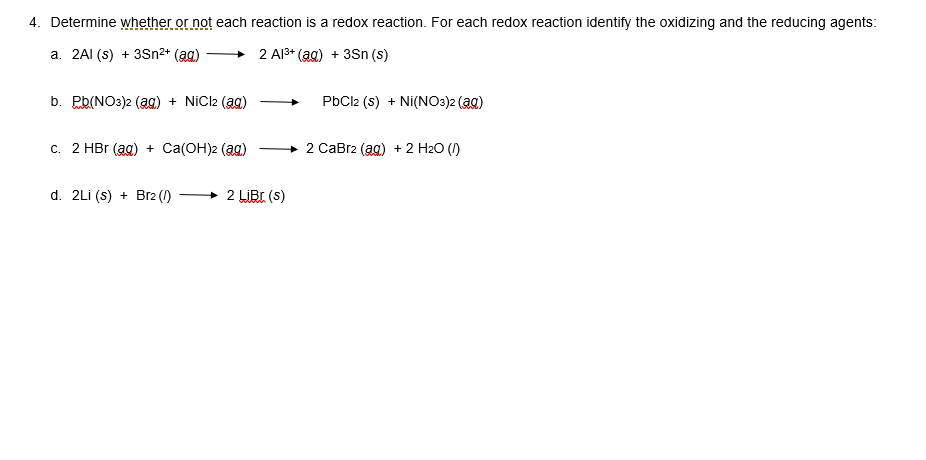
⚗️Complete the following equations (note that the equations are not balanced). Use the activity - Brainly.com

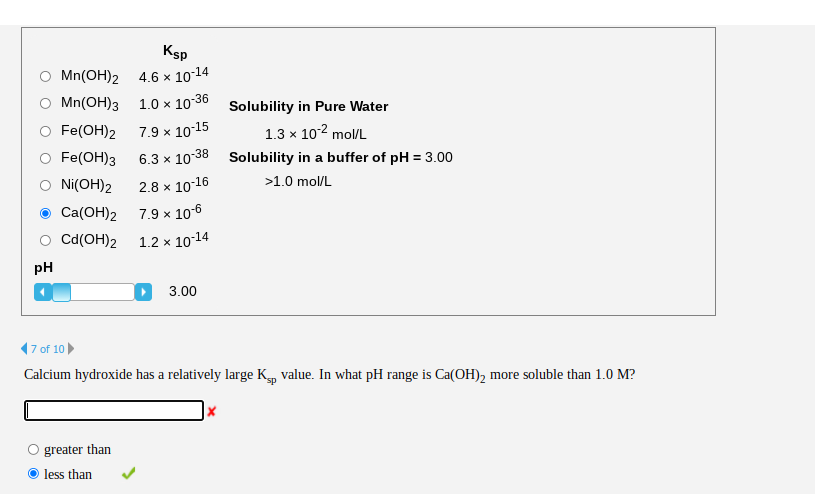
Chemistry lovers - #Metal hydroxides are hydroxides of metals. Metal hydroxides are also known as strong bases. Many common metal hydroxides are made up from hydroxide ions and the ion of the

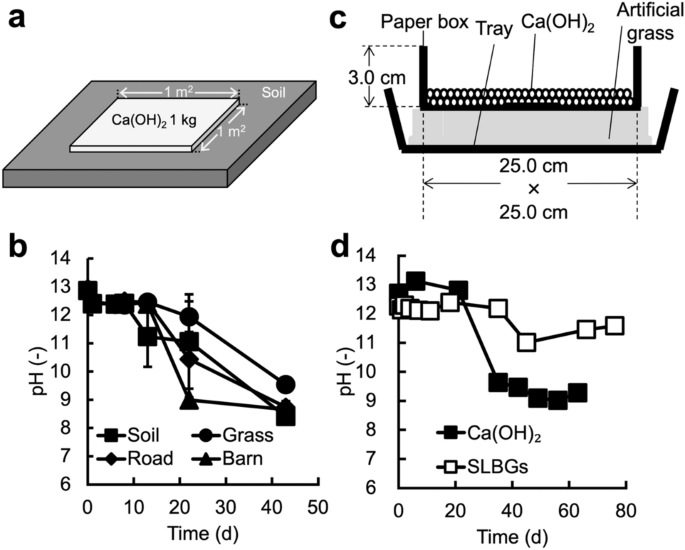
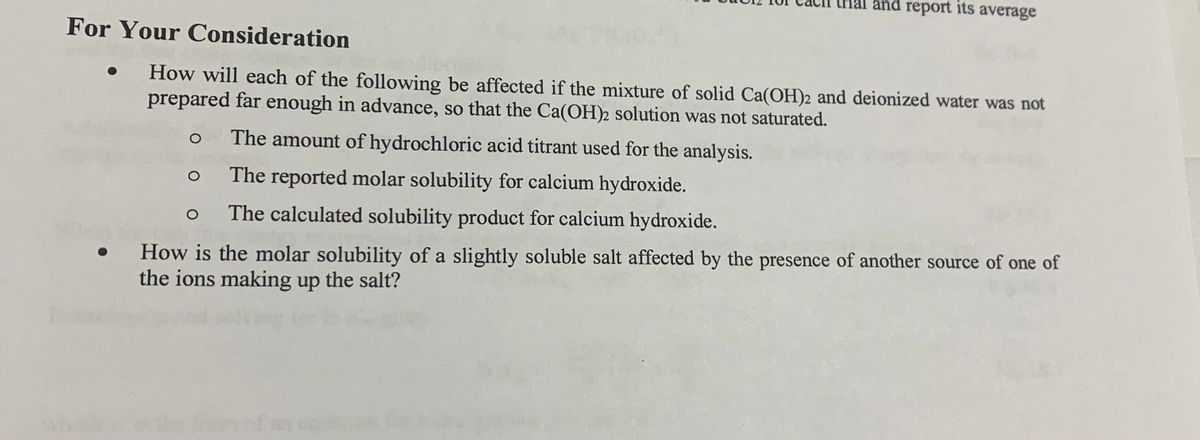
Changes in the quantity of calcium hydroxide (Ca(OH) 2 ) a compound in... | Download Scientific Diagram

Enhancing Hydrogen Evolution Activity in Water Splitting by Tailoring Li+-Ni (OH)2-Pt Interfaces | Science