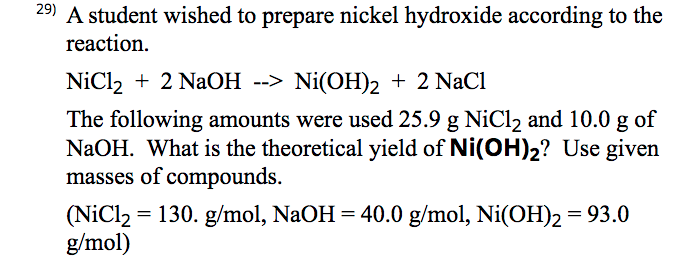Shape-controlled synthesis of Ni(OH)2/NiO nanowalls by surface reaction of Ni foil in aqueous NH4OH - ScienceDirect

Ultrathin Nickel Hydroxide and Oxide Nanosheets: Synthesis, Characterizations and Excellent Supercapacitor Performances | Scientific Reports

science chemistry precipitation reaction nickel chloride | Fundamental Photographs - The Art of Science

Powder XRD patterns of (a) β-nickel hydroxide, (b) oxidation of (a)... | Download Scientific Diagram

Fluorescence spectra of 1 (10 M) in the presence of 400 M of NaOCl,... | Download Scientific Diagram

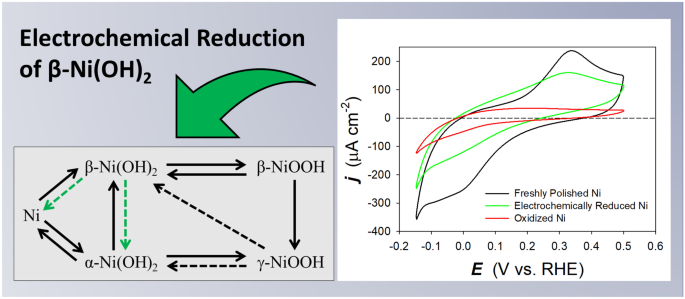
Controllable voltammetric formation of a structurally disordered NiOOH/Ni(OH )2 redox pair on Ni-nanowire electrodes for enhanced electrocatalytic formaldehyde oxidation - ScienceDirect

Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media - ScienceDirect

Scheme 27. (a) 7% aq NaOCl, 90%; (b) H 2 , Raney-Ni, B(OMe) 3 , 100%;... | Download Scientific Diagram

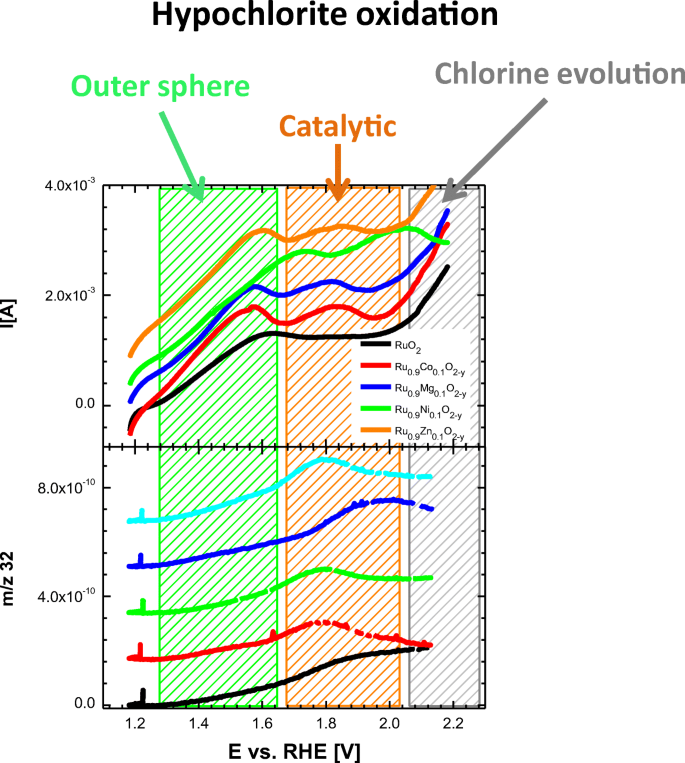
Manufacture characteristics of metal oxide–hydroxides for the catalytic decomposition of a sodium hypochlorite solution - ScienceDirect

Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media - ScienceDirect

Formation of a Ni(OH)2/NiOOH active redox couple on nickel nanowires for formaldehyde detection in alkaline media - ScienceDirect
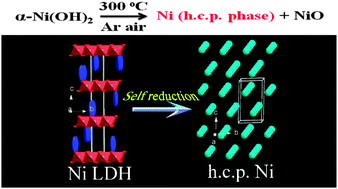
Anomalous self-reduction of layered double hydroxide (LDH): from α-Ni(OH)2 to hexagonal close packing (HCP) Ni/NiO by annealing without a reductant - Chemical Communications (RSC Publishing)
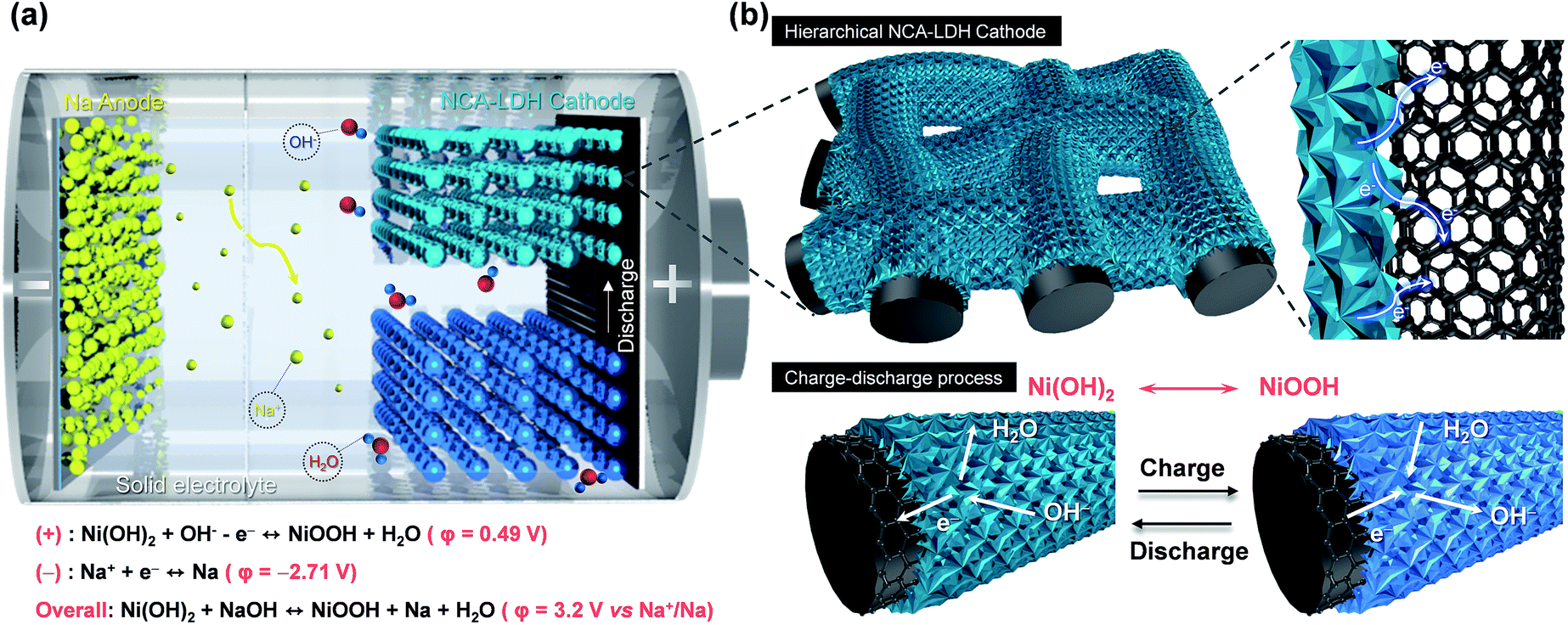
Rechargeable Na/Ni batteries based on the Ni(OH) 2 /NiOOH redox couple with high energy density and good cycling performance - Journal of Materials Chemistry A (RSC Publishing) DOI:10.1039/C8TA10830G

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences